(Image grabbed from DOST Secretary Fortunato dela Peña's Facebook page)
MANILA-- The Food and Drug Administration (FDA) has approved the coronavirus disease 2019 (Covid-19) test kits developed by the University of the Philippines-National Institutes of Health (UP-NIH).
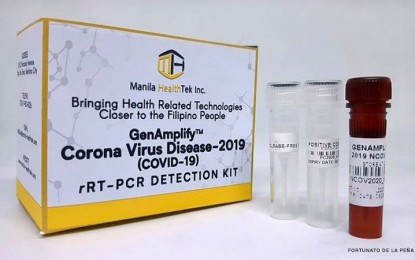
"The Food and Drug Administration (FDA) has now approved the Real-Time PCR for the detection of COVID-19 manufactured by the Manila HealthTek, Inc. for commercial use," the FDA said in an advisory on Friday night.
The UP-developed test kit was previously approved by the FDA for field trial.
Upon the company’s submission of necessary requirements, the FDA issued a certification for the COVID-19 test kit "to be allowed for commercial use".
READ: UP-NIH to do 1K tests per week with coronavirus detection kit
"This is the first locally made PCR based COVID-19 test kit approved by the FDA which was developed in collaboration with the University of the Philippines-National Institute of Health (UP-NIH), funded by the Department of Science and Technology (DOST)," the FDA said.
Aside from the locally-made PCR based test kit, the FDA has also approved one additional rapid test kit on Friday, namely, "Hightop SARS-CoV-2 IgM/IgG Antibody", bringing the total number of Covid-19 approved test kits to 30. (PNA)
