
(File photo)
MANILA – A health official said on Thursday only 25 adverse events following immunization (AEFI) were recorded among the 23,727 minors with comorbidities aged 12 to 17 years old who were inoculated against Covid-19.
In an online media briefing, Health Undersecretary Myrna Cabotaje noted three of the AEFIs were serious cases involving anaphylaxis or severe allergy which required injection of epinephrine and oxygenation.
“’Yung ibang kaso, mild allergies, may konting rashes, may konting sakit sa injection site and many of these, makikita natin sa mga bata even sa (The other cases, mild allergies, some rashes, pain in the injection site and many of these, can be seen in kids even during) measles, rubella [vaccination]”, she said.
Cabotaje added that some children experienced immunization-related anxiety response like fainting and palpitation.
The government is set to start with the vaccination of the pediatric population with or without comorbidities on Nov. 3.
The full implementation of the pediatric vaccination is set to begin on Nov. 5.
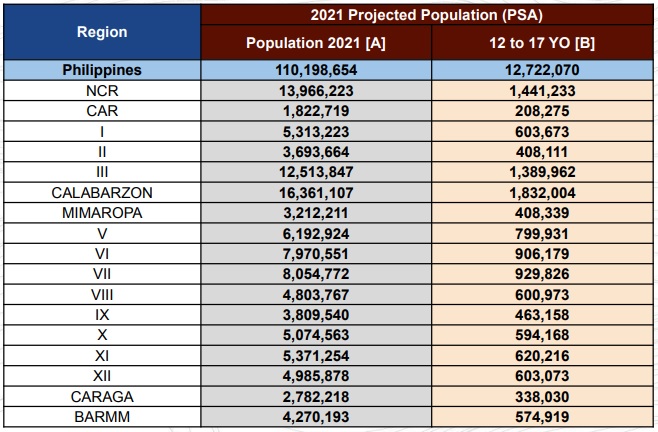
“Ang 12 to 17 years old po ay 12,722,070 individuals sa buong bansa iyan (The total number of 12 to 17 years old nationwide is 12,722,070) and by December our target is to vaccinate at least 80 percent of the target population with two doses,” Cabotaje said.
“Ang binigyan ng Philippine FDA [Food and Drug Administration] ng (The Philippine FDA gave) emergency use authorization to be vaccinated to 12 to 17 years old are Pfizer and Moderna so their intervals are 21 days and 28 days respectively,” she added.
She said the pediatric vaccination will use the regular vaccination sites.
Among the documentary prerequisites needed are identification cards and proof of filiation like birth certificates and baptismal records among others. (PNA)
