
President Rodrigo Duterte (Presidential photo)
MANILA – The prices of drugs and medicines used to address the leading causes of morbidity in the country will now be regulated to ensure that these will remain affordable, according to a new Executive Order (EO) signed by President Rodrigo Duterte on Dec. 7.
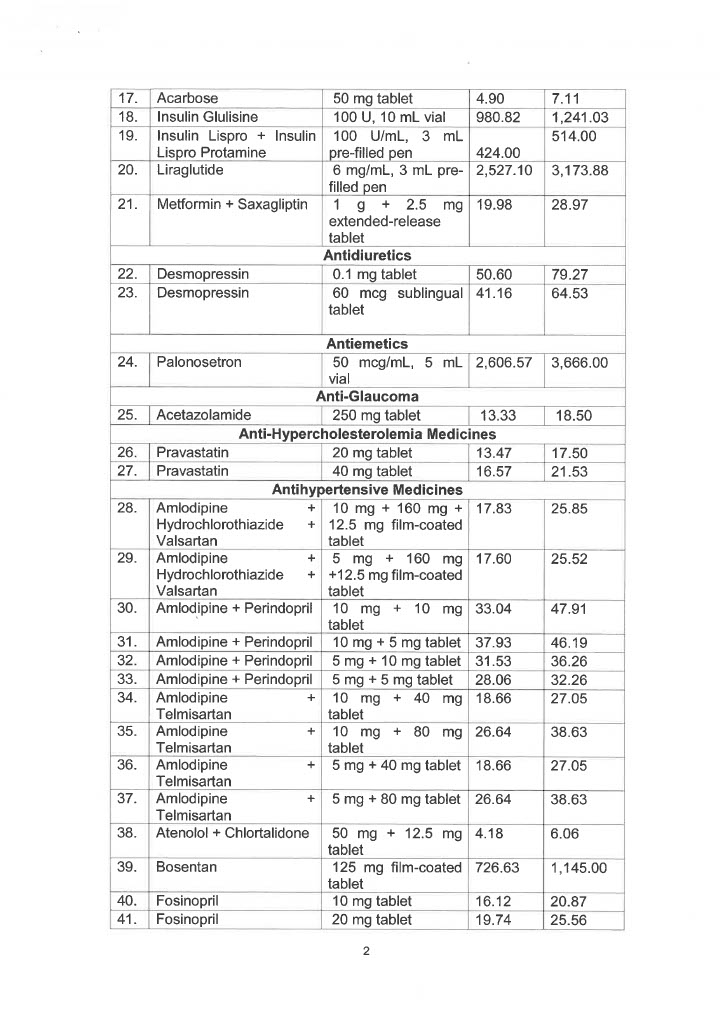
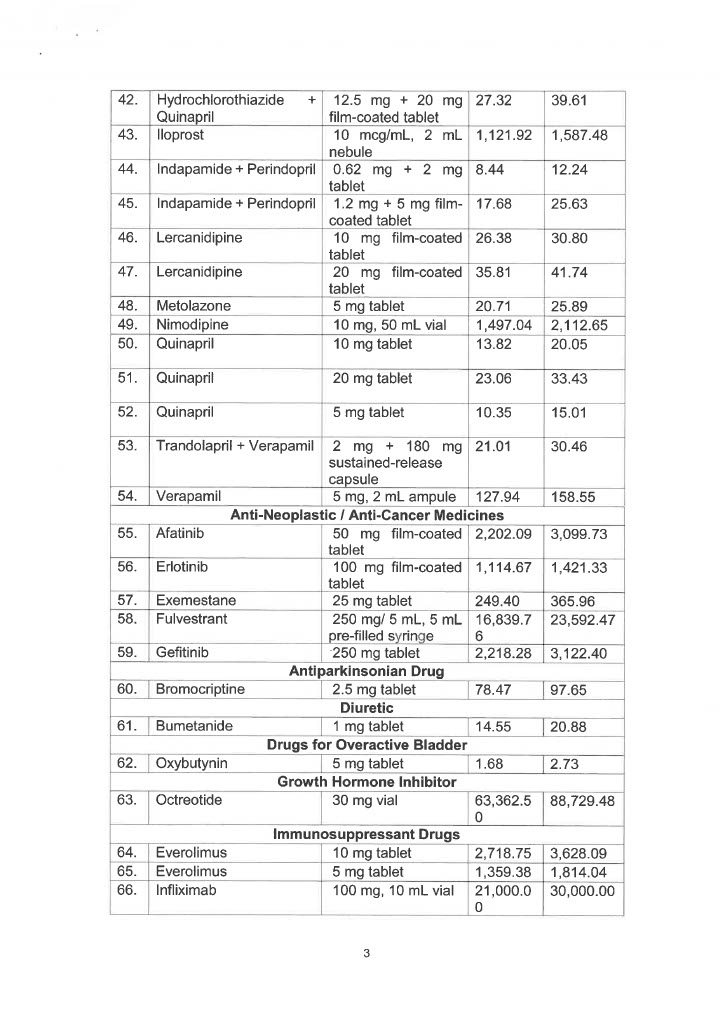
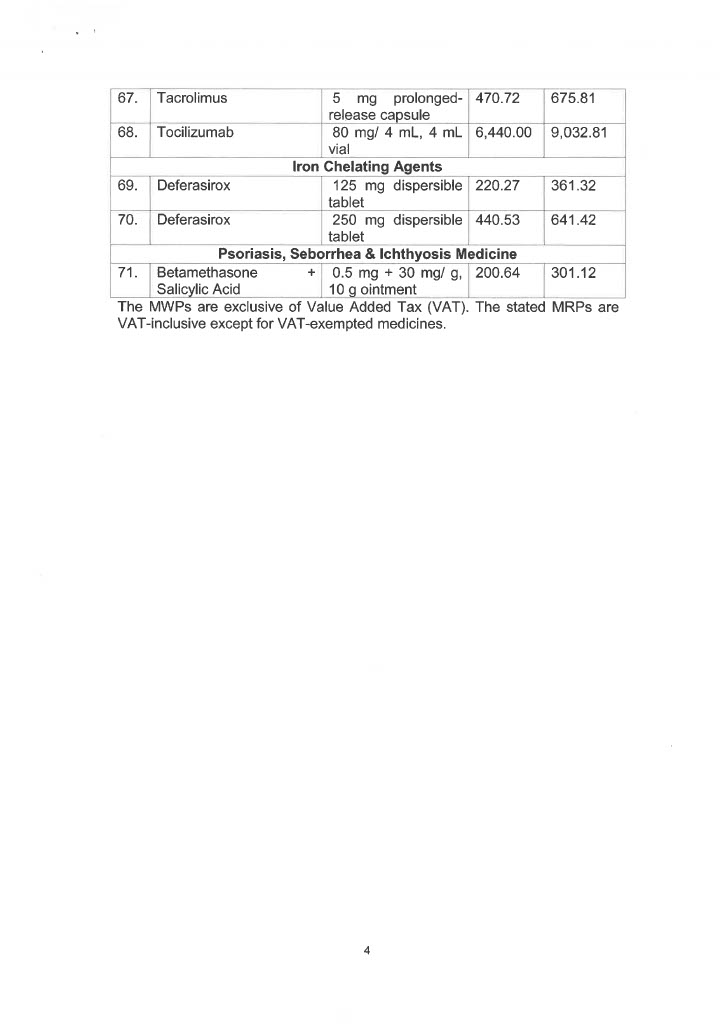
EO 155 sets the maximum retail price (MRP) and/or maximum wholesale price (MWP) on 34 drug molecules or 71 drug formulas used in agents affecting bone metabolism, analgesics, anesthetics, anti-angina, antiarrhythmics, anti-asthma and chronic obstructive pulmonary disease medicines, antibiotics, anticoagulants, anticonvulsants, antidiabetic drugs, antidiuretics, and antiemetics.
Also covered by the EO are drug molecules and formulas utilized in anti-glaucoma, anti-hypercholesterolemia medicines, antihypertensive medicines, anti-neoplastic/anti-cancer medicines, antiparkinsons drugs, drugs for overactive bladders, growth hormone inhibitors, immunosuppressant drugs, iron chelating agents, and psoriasis, seborrhea, and ichthyosis medicines.
"After conducting a price review using the methods of international reference pricing among ASEAN countries, mark-up regulation, and stakeholders' consultation, the TWG [Technical Working Group] recommended, and the DOH [Department of Health] endorsed the imposition of MPR and MWP on the remaining drug formulas of selected drugs and medicines,” the EO read.
"Consistent with the overall strategy under RA (Republic Act) No. 11223 or the "Universal Health Care Act," to improve the access to affordable and quality medicines and reduce health-related out-of-pocket expenses of Filipinos, there is a need to further impose MRP and MWP on other drugs and medicines commonly used for the leading causes of morbidity in the country," it added.
Under EO 155, manufacturers, importers, distributors, wholesalers, traders, or retailer of a drug/medicine are required to label MRP drugs and medicines.
"Every manufacturer, importer, distributor, wholesaler, trader or retailer of a drug/medicine intended for sale shall display the retail price which shall not exceed the MRP. The MRP, preceded by the words "RETAIL PRICE NOT TO EXCEED", and "UNDER DRUG PRICE REGULATION", on a red strip, shall be clearly printed on the label of the immediate container of the drug and medicine and the minimum pack thereof offered for retail. The labeling shall also be applied to drug formulas or medicines under MRP through EO No. 104," the EO further read.
EO 104 created the TWG composed of representatives from the DOH and the Department of Trade and Industry (DTI) for the purpose of reviewing, in consultation with stakeholders, the prices of other drug molecules or drug formulas.
The list of medicines and their corresponding MRPs and/or MWPs, and those covered by EO No. 104, shall be subject to review of the DOH, in consultation with the DTI, six months after the effectivity of this order, and every six months thereafter.
The DOH, in consultation with relevant government agencies, including the DTI and the Philippine Competition Commission, is directed to study and propose measures, including, but not limited to pooled procurement, price negotiation and other mechanisms, which will influence the supply, demand, and expenditure on drugs and medicines, in accordance with RA 9502, and other relevant laws and regulations.
RA 9502 or the "Universally Accessible Cheaper and Quality Medicines Act of 2008" declares it a policy of the State to protect public health and, when public interest or circumstances of extreme urgency so require, to adopt appropriate measures to promote and ensure access to affordable quality drugs and medicines for all.
The DOH shall formulate guidelines for the effective implementation of this Order.
The Presidential Communications Operations Office is directed to provide the necessary support and assistance to the DOH for the dissemination of information relative to this Order.
All other government agencies and instrumentalities including government-owned or controlled corporations, government financial institutions, and state colleges and universities, are hereby directed to provide the necessary support to the DOH in the information dissemination, enforcement, and implementation of the order.
Any violation of the order shall be dealt with in accordance with RA 9502, and other related laws.
Pursuant to Section 19 of RA 9502, the Secretary of Health is directed to investigate alleged violations of the MRP and/or MWP under this order, impose administrative fines and penalties, and call upon and deputize government entities for assistance necessary to carry out the purpose of this order.
Under Section 19 (D) of RA 9502, the Secretary of Health has the power to impose administrative fines of not less than PHP50,000 nor more than PHP5 million.
With a non-extendable period of 90 days from the effectivity of the order, existing inventory stock shall be disposed of at prevailing prices. Thereafter, regardless of the status of the existing stock, the MRP and/or MWP under the order shall be strictly implemented.
Below is the list of MRP/MWPs on 34 drug molecules or 71 drug formulas: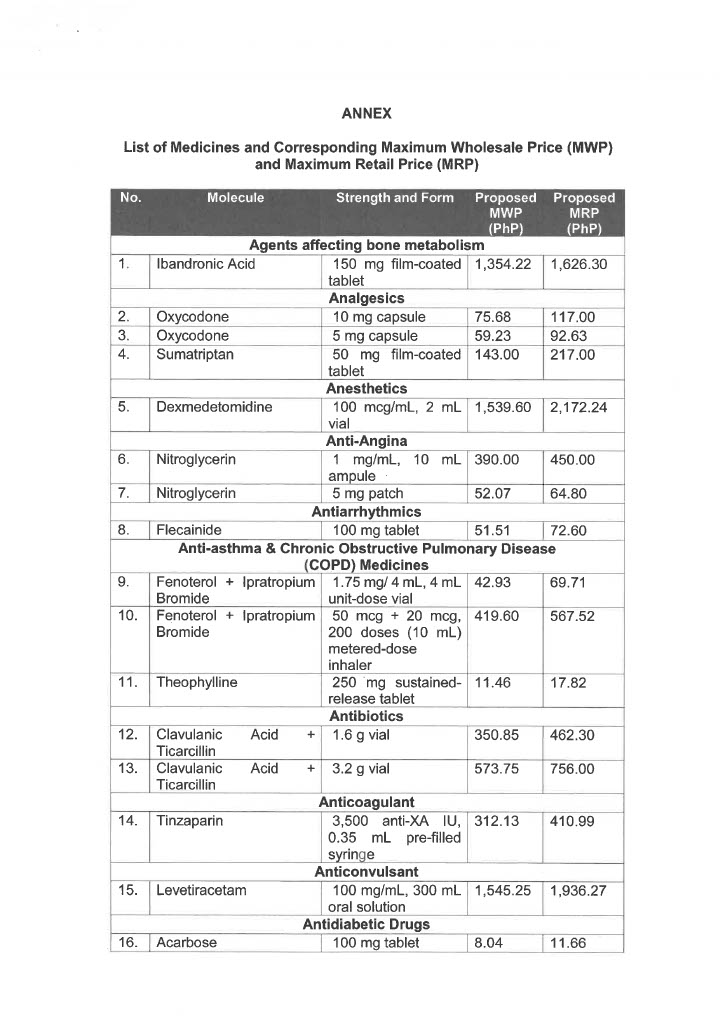
(PNA)
