(Photo courtesy of UN)
MANILA – There is no Food and Drug Administration (FDA) approved and authorized vaccine against monkeypox but studies have shown that smallpox jabs work through "cross-protection", the Department of Health (DOH) said on Thursday.
In a Viber message to reporters, the DOH reported that it is important to note that the monkeypox case is not infectious during the incubation period of five to 21 days.
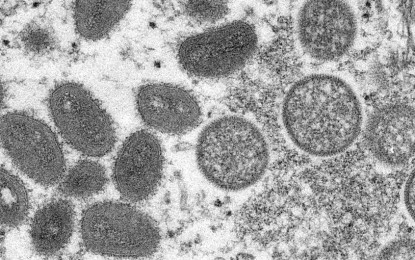
“The case may start to become infectious during the prodromal stage wherein the case develops the first symptoms including fever, malaise, headache, sometimes sore throat and cough, and lymphadenopathy or swollen lymph nodes,” the department said.
Citing data from United States Centers for Disease Control and Prevention, the DOH said lesions will develop in the mouth and on the body after the prodromal stage.
“A person is considered contagious from the onset of the enanthem (rash) through the scab stage. Once all scabs have fallen off a person is no longer contagious,” the DOH said in the same message.
Observance of the minimum public health standards – wearing of best-fitted mask, ensuring good airflow, handwashing, and physical distancing – can prevent monkeypox transmission and also protection from coronavirus disease 2019 (Covid-19).
Furthermore, the DOH is intensifying screening at our borders and ensuring that surveillance systems are actively monitoring the situation, the same message added.
Earlier, DOH–Technical Advisory Group member, Dr. Edsel Salvaña said the country does not have a stockpile of vaccines for monkeypox but the government has contacts with the Centers for Disease Control and other agencies in case procurement would be needed. (PNA)
