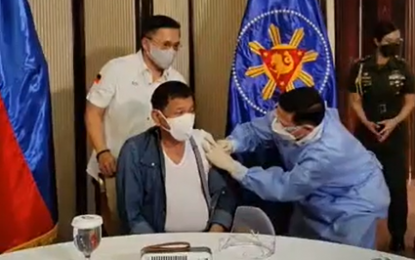
STILL TO GET 2ND DOSE. President Rodrigo Duterte receives his first shot of a Sinopharm Covid-19 vaccine on Monday night (May 3, 2021). Malacañang on Thursday (May 6) said Duterte is still scheduled to receive his second dose of the vaccine despite his order to pull out Sinopharm jabs over concerns that the vaccine has yet to receive an emergency use authorization (EUA) from local regulators. (Screenshot from Senator Bong Go’s Facebook livestream)
MANILA – Despite ordering the withdrawal of China’s Sinopharm Covid-19 vaccines in the country, President Rodrigo Duterte is still scheduled to receive his second dose of the jab, Malacañang said on Thursday.
Presidential Spokesperson Harry Roque made this statement after Duterte on Wednesday evening said he wanted Sinopharm pulled out over concerns that it has yet to receive an emergency use authorization (EUA) from the Food and Drug Administration (FDA) of the Philippines.
“Hindi ibabalik yung pang second dose ni Presidente para matapos niya ang second dose niya (The second dose for the President will not be returned so that he can complete his vaccination),” he said in a Palace press briefing.
Duterte is expected to receive his second dose of Sinopharm 21 to 28 days after his first dose administered to him by Health Secretary Francisco Duque III last Monday.
Unless Sinopharm receives an EUA, Roque said Duterte is bent on having the vaccines pulled out to erase controversy hounding his vaccination.
“Ang sabi ni Presidente, para mawala na ang kontrobersiya dahil marami ngang nagreklamo na kinuha niya ang Sinopharm maski meron naman po itong compassionate use e para mawala na yung ganyang mga kritisismo at habang siya pa lang ang gumagamit ng 1,000 na pagbakuna, siyempre mas mabuting ibalik na muna sa Tsina (To erase controversy because many are complaining that he got Sinopharm even if it has a compassionate use permit, he thinks it’s better to return them to China),” he said.
He, however, said Duterte may change his stand should Sinopharm be cleared for emergency use.
“Tignan po natin kung anong mangyari because who knows? Since the EUA naman has been given to Sinopharm in 25 other countries baka tama po kayo, mapabilis naman po yung proseso ng pagkuha ng EUA (Let’s see what happens because who knows? Since the EUA has been given to Sinopharm in 25 other countries, maybe you’re right, the process of getting an EUA may be hastened),” he added.
Some 1,000 doses of Sinopharm donated by China to the Philippines were issued with a compassionate use permit intended only for the Presidential Security Group (PSG) hospital.
“Sa donasyon (When it comes to donations), it is found in all legal systems that’s an act of beneficence, generosity of the donor. So wala naman pong usapin na requirement para mag-donate (So there is no issue about the requirement to donate),” he said.
Sinopharm agent
Roque also raised the possibility of Sinopharm sending a representative to hasten the process of filing an application for an EUA.
To recall, he earlier described the process of Sinopharm applying for an EUA as “grossly delayed”.
“Kaya po nahihirapan o nagtatagal yung proseso kasi kinakailangan magtalaga ng representante ang Sinopharm kung sino talaga ang maghahain ng papeles dito sa Pilipinas sa FDA (The reason why the process is taking too long is because there is a need for Sinopharm to designate a representative to file papers before the FDA),” he said.
Once Sinopharm sends in an agent, he expressed hope that EUA will be cleared for emergency use in the Philippines.
“Panatag naman po ang loob natin na maaprubahan din ang EUA ng Sinopharm dahil Sinopharm is in use po in 25 countries worldwide at nakakuha na rin siya ng EUA in 25 other countries (We are confident that the EUA of Sinopharm will be approved since Sinopharm has been given EUA and is now being used in 25 countries worldwide),” he added.
FDA Director-General Eric Domingo earlier said Sinopharm has yet to submit an official application for EUA but two local distributors have manifested their intent to apply for EUA.
‘Can’t be choosy’
With the arrival of 15,000 doses of the Sputnik V Covid-19 vaccines from Russia, Roque reminded the public not to be “choosy” over which vaccine brand to get.
He said only front-line healthcare workers have the luxury of selecting their preferred vaccine brand.
“Sa mula’t mula po ganyan ang polisya natin. Hindi po pupuwede na parang restaurant ‘Ang gusto ko ganito, ang gusto ko ganito.’ Hindi po. Kung ano yung naririyan, ‘yun po ang ibibigay sa inyo at tanging front-liners lang po ang binigyan natin ng ganyang karapatan (That has been our policy from the very start. You can’t act like you’re in a restaurant and say ‘I want this, I want that’. No. Whatever is there, that is the vaccine we will give you and only front-liners have the luxury of choosing their preferred brand),” he said.
Besides Sputnik V, the Philippines has two other vaccine brands in its inventory that have been approved for emergency use namely “CoronaVac” from China’s Sinovac Biotech and “AstraZeneca” from a British-Swedish manufacturing company of the same name. (PNA)
