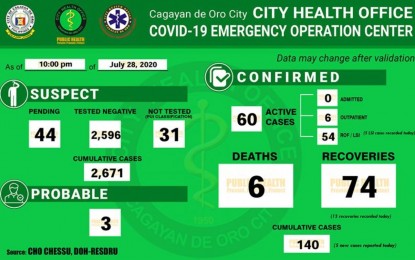
The Cagayan de Oro City Covid-19 situation report as of 10 p.m. July 28, 2020.
CAGAYAN DE ORO CITY--The city's coronavirus disease 2019 (Covid-19) recoveries have already surpassed the number of active cases, local officials said Wednesday.
Dr. Lorraine Nery, acting head of the City Health Office (CHO), said that Wednesday's 13 new recoveries brought the total recovered patients here to 74 since the pandemic was declared in March.
The figures, Nery said, dwarfed the 60 active local cases as of July 28, representing around 53 percent recovery rate from the total 140 cases recorded here since March.
Among the new 13 recoveries include the last patient from Dabatian, Barangay Carmen, who tested negative on her seventh and last swabbing since quarantined in the city patient care center for the last 49 days.
As of Wednesday, the Covid-19 deaths remained at six.
"We no longer have admissions in NMMC (Northern Mindanao Medical Center), our Covid-19 referral center, from these 60 cases, although our patients are in our TTMFs (temporary treatment and monitoring facilities)," she said.
Dr. Tedulfo Joselito Retuya, CHO's resident epidemiologist, said that the 60 patients currently housed in the care facilities--including the new five patients tagged as locally stranded individuals and repatriated overseas Filipinos--were "generally asymptomatic".
"There were some, one or three of them, who reported having minor allergic issues and were already attended by the staff," Retuya said.
Mayor Oscar Moreno attributed the increase of recovered patients to the strict implementation of health protocols and patient care initiated by the city's medical front-liners and staff.
"We can already see the trend (of cases) here. We are aware of what's happening. The challenge now is how do you preempt the problem, or how do you solve the problem," Moreno said.
Proactive action
Meanwhile, NMMC said it currently maintains three anti-viral drugs that can help alleviate the conditions of patients infected with Covid-19.
Other than Remdesivir, other drug variants include Tocilizumab and Lopinavir-Ritonavir, which are all approved by the Department of Health's (DOH) Food and Drug Administration (FDA), said Dr. Bernard Julius Rocha, NMMC medical specialist and liaison officer of NMMC, on Tuesday.
Rocha said NMMC was "lucky" to have DOH purchased the drugs for the hospital to cope up with its responsibility being Northern Mindanao's referral hospital for Covid-19 cases.
He said the drugs were part of the clinical trials set by the World Health Organization (WHO), of which the Philippines is a participant.
The drugs, with an average cost of PHP9,000 to PHP37,000 per vial, will be administered free to patients showing severe or critical conditions because of their co-morbidities, Rocha said.
"Procurement of medications is very hard due to availability. Furthermore, these drugs are approved for the use of a specific hospital only to address specific cases for Covid since they are approved by FDA more so the use of Remdisivir," he added.
As of Wednesday, NMMC has two Covid-19 patients, all admitted in a building exclusively for Covid-19 cases that can accommodate more than 90 patients and can cater to hundreds more as soon as renovations are finished, NMMC officials said.
Rocha made it clear that the three drugs are no cure per se, but have initially been proven to lessen the time of hospitalization of patients and help in their physical improvement.
He said that a portion of the Remdesivir stocks in NMMC was given to Amai Pakpak Medical Center in Marawi City, based on FDA's "compassionate use" policy.
An article published by the New England Journal of Medicine (NEJM) on May 7 cited an initial study showing no improvement in Covid-19 patients who were administered with Lopinavir-Ritonavir.
Some medical practitioners who wrote to the journal editor as a response to the study, however, said the findings may be premature to conclude and more time for observation should be given.
On July 4, the WHO announced that it will discontinue the use of Lopinavir-Ritonavir, along with Hydroxychloroquine, based on the recommendations made by the Solidarity Trial’s International Steering Committee.
Rocha said that NMMC's 140-vials of Lopinavir-Ritonavir will still not go to waste because it's a combination drug primarily intended for HIV cases.
"The treatment was part of the trial to find out if it is acceptable to use for Covid-19. If it was dropped, then it would be used for our existing HIV program, which the drug was really meant to be used for. This is why we have the stocks already because of our ongoing HIV program, and we are the first hospital in Mindanao with this HIV program. Same with our stocks of hydroxychloroquine it goes back to be used for patients with malaria, lupus, among others," he said.
Also published by NEJM on June 11, the study funded by Gilead Sciences showed that Remdesiver resulted in "clinical improvement" for patients subjected to the study. The study, however, added that it will still require randomized, placebo-controlled trials of Remdesivir therapy so that researchers can measure the drug's efficacy.
For Tocilizumab, a study published on June 24 by The Lancet journal showed a possibility that the drug may reduce the risk of Covid-19 patients from dying or undergoing "invasive mechanical ventilation."
Dr. Joanna Sabal, head of NMMC's Emergency Operations Center for Covid-19, said that because the supply of the three drugs are limited, the administration of these to the patients should be based on the severity of the case.
"We have to think the efficient use of that medication (Remdesivir), because we know that globally, there's a shortage of it and it's needed in a lot of (Covid-19) cases. We do have stocks, but the cases go up, then those stocks will not be really that much, to be honest," she said. (PNA)
